
Robert Reed Burn, Introduction to Nuclear Reactor Operation, 1988.
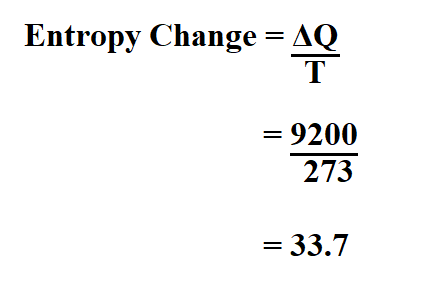
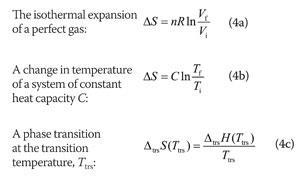
Physics of Nuclear Kinetics. Addison-Wesley Pub. Nuclear and Particle Physics. Clarendon Press 1 edition, 1991, ISBN: 978-0198520467 Nuclear Reactor Engineering: Reactor Systems Engineering, Springer 4th edition, 1994, ISBN: 978-0412985317 Stacey, Nuclear Reactor Physics, John Wiley & Sons, 2001, ISBN: 0- 471-39127-1. Baratta, Introduction to Nuclear Engineering, 3d ed., Prentice-Hall, 2001, ISBN: 8-1. Lamarsh, Introduction to Nuclear Reactor Theory, 2nd ed., Addison-Wesley, Reading, MA (1983). The fact that the absolute value of specific entropy is unknown is not a problem, however, because it is the change in specific entropy (∆s) and not the absolute value that is important in practical problems. For example, the specific entropy of water or steam is given using the reference that the specific entropy of water is zero at 0.01☌ and normal atmospheric pressure, where s = 0.00 kJ/kg. Normally, the entropy of a substance is given with respect to some reference value. In general, specific entropy is a property of a substance, like pressure, temperature, and volume, but it cannot be measured directly. Because entropy tells so much about the usefulness of an amount of heat transferred in performing work, the steam tables include values of specific entropy (s = S/m) as part of the information tabulated. M = mass (kg) T-s diagram of Rankine CycleĮntropy quantifies the energy of a substance that is no longer available to perform useful work. It equals to the total entropy (S) divided by the total mass (m). The specific entropy (s) of a substance is its entropy per unit mass. Engineers use the specific entropy in thermodynamic analysis more than the entropy itself. The entropy can be made into an intensive, or specific, variable by dividing by the mass.


 0 kommentar(er)
0 kommentar(er)
